This probe is available from Sigma and Cayman Chemical.
Its negative control (TH-263) is available for purchase from Cayman Chemical.
| Probe | Negative control | |
 |
|  |
TH-257 |
| TH-263 |
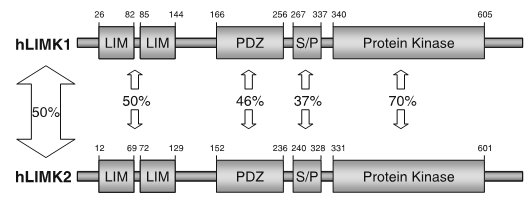
LIM kinases belong to the family of cytoplasmic tyrosine-like kinases with dual specificity (serine/threonine and tyrosine). However, known LIMK substrate are usually phosphorylated at serine and threonine residues LIM kinases comprises LIM kinase 1 (LIMK1) and LIM kinase 2 (LIMK2) which show 50% sequence identity in human. Both LIMK1 and LIMK2 present with a unique domain organization containing two N-terminal LIM domains, a PDZ domain, a proline/serine-rich domain and a C-terminal kinase domain [1].

Both proteins are expressed widely in embryonic and adult tissues, but show some cell-type specific expression. Accordingly, the two kinases have overlapping functions, but appear non-redundant. Knockout studies in mice show that LIMK1 is required for development of the central nervous system [2], whereas LIMK2 knockout impairs the activity of testicular germ cells [3].
LIMKs are effectors of cell morphology and motility and apoptosis by regulating the actin cytoskeleton. The LIMKs signal downstream from Rho GTPases and are activated by phosphorylation of the activation loop by upstream kinases, including Rho kinase (ROCK), PAK1/2/4 and MRCKα. The best characterized LIMK substrates are cofilin1 (non-muscle cofilin), cofilin2 (muscle cofilin) and destrin (actin depolymerizing factor, ADF). Phosphorylation of cofilin serine-3 inactivates the actin severing ability promoting F-actin polymerization, stress fibre formation and focal adhesion formation [4].
LIM kinases can shuttle between the cytoplasm and the nuclear compartment of a cell, a process tightly regulated by association with other partners such as p57kip2 and phosphorylation in the activation segment by PAK kinases [1]. Inhibition of LIMK hyper-stabilizes mitotic spindles inducing a G2/M cell cycle block suggesting an important role for these kinases in microtubule dynamics [5].
Increased phosphorylation of LIMK1 has been reported in neurons in areas affected with Alzheimer Disease [6]. LIM kinases play important roles in cancer metastasis like highly invasive prostate and breast cancer, which is reversed by gene silencing [7, 8]. LIMK1 overexpression is also found in malignant melanoma, as well as most tumour cell lines. Other applications for LIMK inhibitors are open-angle glaucoma [9]. In addition, LIMK1 interacts with the long isoform of the type II bonemorphogenetic protein (BMP) receptor contributing to the pathology of Fragile X syndrome, a common inherited form of intellectual disability [10].
TH-257 is a chemical probe for LIMK1 and LIMK2. TH-257 is an allosteric inhibitor targeting a binding pocket induced by an αC and DFG-out conformation. It potently inhibits cofilin phosphorylation with an IC50 of 84 nM for LIMK1 and 39 nM for LIMK2 in a RapidFire MS assay. TH257 is exquisitely selective and no significant activity against the wider kinome has been observed in the KINOMEscan assay (Dx) at 1 μM (IC50 >> 50 % inhibition). In a life cell NanoBRET assay (Promega) TH257 has an IC50 of 250 nM against ectopically expressed full-length LIMK1 and 150 nM LIMK2, respectively.
A chemically related negative control compound, TH-263, is provided.
| Probe | Negative control | |
 |
|  |
TH-257 |
| TH-263 |
| Physical and chemical properties for TH-257 | |
| Molecular weight | 422.2 |
| Molecular formula | C24H26N2O3S |
| MollogP | 5.138 |
| PSA | 57.08 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 10 |
| No. of hydrogen bond acceptors | 6 |
| No. of hydrogen bond donors | 1 |
| Physical and chemical properties for TH-263 (Negative Control) | |
| Molecular weight | 380.1 |
| Molecular formula | C21H20N2O3S |
| MollogP | 3.636 |
| PSA | 66.41 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 8 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 2 |
SMILES:
TH-257: CCCCN(C(C1=CC=C(S(NC2=CC=CC=C2)(=O)=O)C=C1)=O)CC3=CC=CC=C3
TH-263: O=S(C1=CC=C(C=C1)C(NCC2=CC=CC=C2)=O)(NCC3=CC=CC=C3)=O
InChI:
TH-257: InChI=1S/C24H26N2O3S/c1-2-3-18-26(19-20-10-6-4-7-11-20)24(27)21-14-16-23(17-15-21)30(28,29)25-22-12-8-5-9-13-22/h4-17,25H,2-3,18-19H2,1H3
TH-263: InChI=1S/C21H20N2O3S/c24-21(22-15-17-7-3-1-4-8-17)19-11-13-20(14-12-19)27(25,26)23-16-18-9-5-2-6-10-18/h1-14,23H,15-16H2,(H,22,24)
InChIKey:
TH-257: VNCIWNGCMAKKEO-UHFFFAOYSA-N
TH-263: QDGVJMITKNOVTP-UHFFFAOYSA-N