The probe UNC1999 is available from Cayman Chemical, Sigma and Tocris.
| Probe | Negative control | |
 |
|  |
UNC1999 |
| UNC2400 |
| Biotinylated UNC1999 |
 |
UNC2399 |

The histone H3-lysine 27 (H3K27) methyltransferase EZH2 plays a critical role in regulating gene expression, and its aberrant activity is linked to the onset and progression of cancer. A collaboration between the SGC and the Center for Integrative Chemical Biology and Drug Discovery (CICBDD) at the University of North Carolina at Chapel Hill has resulted in the discovery of UNC1999. UNC1999 inhibits EZH2 with an IC50 of 2nM and is over 1000-fold selective for other HMTs except EZH1 (22-fold selectivity). UNC1999 inhibits H3K27 methylation in MCF10A cells with an IC50 of 124nM as measured by immunofluorescence. A dimethylated version, UNC2400, was also used as a negative control compound in key experiments.
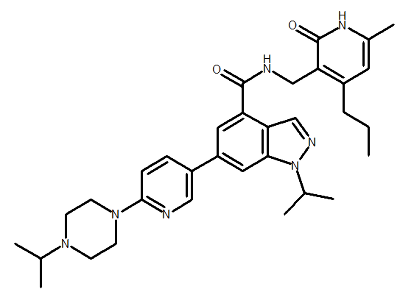
| UNC1999 |
 |
N-[(6-methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl]-1-(propan-2-yl)-6-{6-[4-(propan-2-yl)piperazin-1-yl]pyridin-3-yl}-1H-indazole-4-carboxamide Click here to download the SD file |
| Physical and chemical properties | |
|---|---|
| Molecular weight | 569.74 |
| Molecular formula | C33H43N7O2 |
| IUPAC name | N-[(6-methyl-2-oxo-4-propyl-1,2-dihydropyridin-3-yl)methyl]-1-(propan-2-yl)-6-{6-[4-(propan-2-yl)piperazin-1-yl]pyridin-3-yl}-1H-indazole-4-carboxamide |
| logP | 4.01 |
| PSA | 95.39 |
UNC1999 is selective for EZH2 over 15 other methyltransferases and proteins in other target classes

UNC1999 is Cellularly Active

A. Treatment of MCF10A cells for 3 days with UNC1999 shows a dose-related decrease in H3K27me3 which is unrelated to cell viability.
B. UNC2400 did not show any dose-related decrease in H3K27me3.
UNC1999 Kills DB cells with Y641N mutation

DB cells harbor the EZH2 Y641N mutation
C. 8-day treatment of UNC1999 but not UNC2400 kills DB cells.
D. Western blotting of EZH2, total H3 and H3K27me3 following 3-day treatment of UNC1999 shows no change in H3 or EZH2, but significant change in H3K27me3
An Orally Bioavailable Chemical Probe of the Lysine Methyltransferases EZH2 and EZH1
Kyle D. Konze, Anqi Ma, Fengling Li, Dalia Barsyte-Lovejoy, Trevor Parton, Christopher J.MacNevin, Feng Liu, Cen Gao, Xi-Ping Huang, Ekaterina Kuznetsova, Marie Rougie, Alice Jiang, Samantha G. Pattenden, Jacqueline L. Norris, Lindsey I. James, Bryan L Roth, Peter J. Brown, Stephen V. Frye, Cheryl H. Arrowsmith, Klaus M. Hahn, Gang Greg Wang, Masoud Vedadi, and Jian Jin.
ACS Chem. Biol., 2013, 8 (6), pp 1324–1334
DOI: 10.1021/cb400133j • Publication Date (Web): 08 Apr 2013